Горячая линия Леон
Горячая линия — очень удобный способ связи с любой компанией. К сожалению, у Леона подобного варианта общения с клиентами попросту нет. Как же тогда обратиться за помощью? Читайте ниже.
Горячая линия БК Леон
Увы, но вы нигде не найдете номера телефона горячей линии букмекерской конторы Леон. Компания не предоставляет такой вид связи. Соответственно, подобной информации нет нигде: ни на официальном сайте, ни в разделе «Контакты», ни в мобильном приложении, ни где-либо в другом месте. Как следствие, активным игрокам и потенциальным клиентам leon.ru приходится использовать другие методы для решения вопросов, которые возникли в процессе совершения ставок на спорт. К слову, оффшорный букмекер Leonbets находится в аналогичной ситуации. БК не использует классический способ общения через телефон.
Так как же связаться с саппортом БК Леон?
Если кратко, то организация предлагает пользователям два похожих между собой метода:
- Отправить электронное письмо на почту (help@leon.ru или info@leon.ru).
- Написать сообщение, используя форму обратной связи.
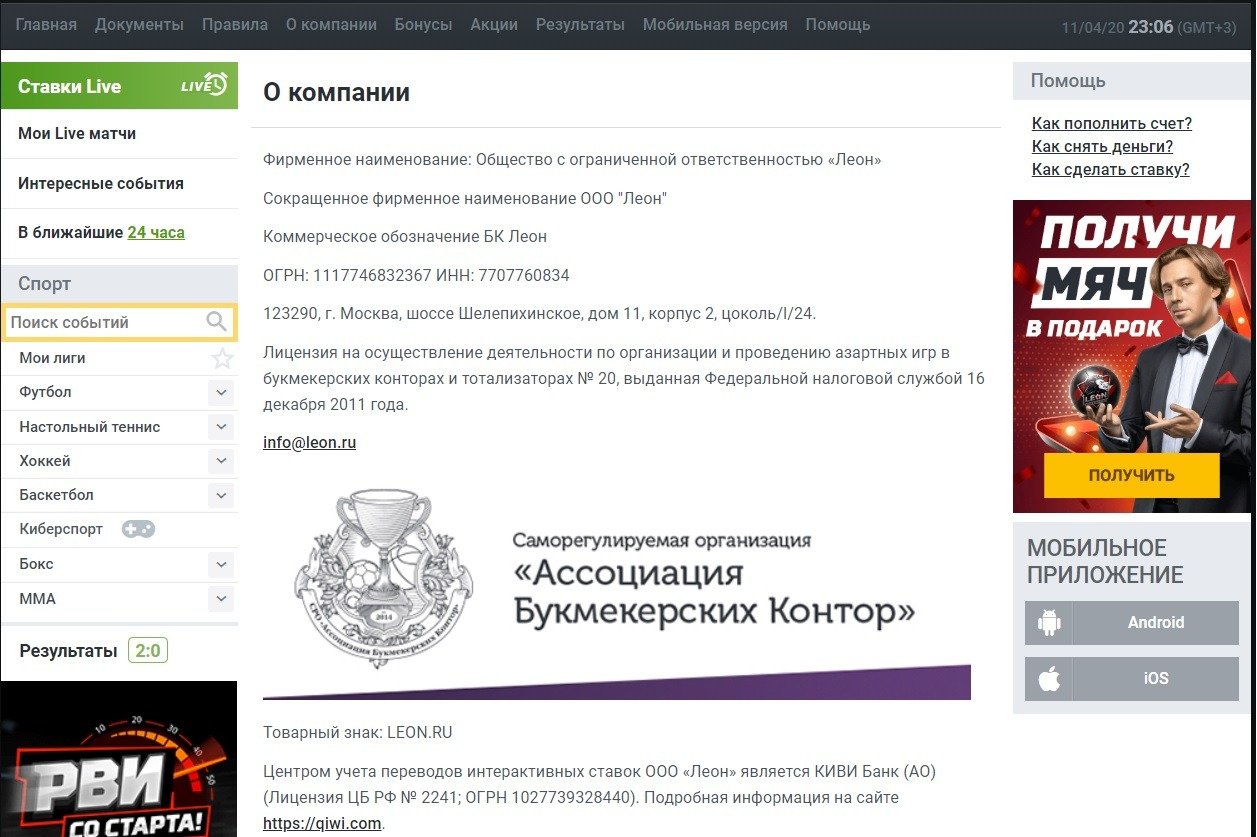
Форма обратной связи доступна в разделе «Помощь» главного меню. И в первом, и во втором случаях, принудительная регистрация не нужна. Отправить заявку через обратную связь может даже обычный посетитель сайта.
Кроме этого, в Москве размещен офис компании. Адрес, а также другая полезная информация, к примеру, о лицензии, прописана в разделе «О компании».
Резюме
Конечно, хотелось бы больше различных вариантов, которые бы ускорили обработку заявки. К примеру, онлайн чат здесь также не предусмотрен. Будем надеяться, что легальный букмекер в будущем прислушается к беттерам и увеличит количество способов, чтобы обратиться в support официального букмекера РФ. И напоследок, оставляя отзывы в Интернете, игроки не только довольны работой саппорта, но и часто отмечают приятный бонус от букмекера. Напомним, вознаграждения для новых клиентов составляет до 20 000 рублей на первый депозит.
Иконка лайв-чата размещена на сайте betboom.ru в правом нижнем углу. Оператор реагирует на запрос в течение минуты. В любой момент диалоговое окно можно закрыть, чтобы оно не мешало серфингу по сайту.
Архитектурные студии, Где заказать технический план, Архитектурные бюро, Архитектурно-строительные компании, Компании по жилищному строительству, Гражданское строительство, Возведение офисных зданий, Компании по строительству административных зданий, Строительство административных и офисных зданий, Строительство гаража под ключ, Построить гараж, Строительство стен и перекрытий для гаража, Компании по строительству гаражей, Строительство гаражей под ключ, Компании по строительству дачных домов и коттеджей, Строительство коттеджных домов, Строительство дач, Строительство дачных домов, дач и коттеджей, Магазины кровельных материалов, Продажа кровельных материалов, Производственные здания, Производственные организации, Промышленные заводы, Производственные помещения, Промышленные предприятия, Производственные компании и фирмы, Производственные предприятия и организации, Строительные конструкции, Где заказать профлист, Доставка профнастила, Где купить профнастил, Магазины профнастила
Скачать мобильную версию бк leon для андроид стоит всем, кто хочет в любое время иметь доступ к любимому букмекеру. И особенно новичкам, которые хотят попробовать свою удачу. Все новые пользователи, зарегистрировавшиеся через программу, получают подарок – 500 рублей фрибета. То есть для начала можно поставить подарочные деньги и проиграть будет совсем нестрашно.
В этом блоке собрана статистическая информация о командах Ванкувер Уайткэпс и Леон, основанная на анализе результатов их последних матчей.
Последние десять лет жили в США, писали на русском языке в жанрах современной научной фантастики, фэнтези и сказки. Их книги переведены и издаются в Америке, Великобритании, Германии, Франции, Италии, Испании, Бразилии, Китае и других странах.
В распоряжении гостей – фруктовый сад с мандариновыми и апельсиновыми деревьями, инжиром и фейхоа, несколько отдельных зон отдыха, мангал для любителей приготовления.
Стоит также отметить, что использование фрибета не будет доступно тем лицам, которые не могут пройти регистрацию в букмекерской конторе Леон. К ним относятся несовершеннолетние лица, а также граждане каких-либо других государств, кроме России. Также бесплатные фрибеты недоступны для тех игроков, которые уже имеют верифицированный аккаунт в рассматриваемой букмекерской конторе. Создавать несколько аккаунтов категорически запрещено.
«Леон» застрахует ваши ставки на российский футбол! Заключайте пари на матчи и получайте половину от суммы на бонусный счет, если ставка не сыграет.
В случае успеха сумму чистого выигрыша за вычетом фрибета БК «Леон» закинет на ваш бонусный баланс. Для отыгрыша необходимо сделать две ставки на сумму от 500 рублей с коэффициентом не ниже 2.00 в течение 48 часов с момента зачисления выигрыша вашего фрибета. После выполнения всех условий клиент может перевести денежные средства на основной счет.
Также вместе с Леонбетс Новый Версия ищут:
- Леон Букмекер Автоматы
- Рейтинг Бк Леон
- Букмекерская Контора Леон Зайти
- Бк Leon Ru
- Бк Леон Просят Фото Банковских Карт
- Бк Леон Superkod Space